
On May 5th, the biggest public medical trust institution Barts Health in UK did performance verification on SARS-CoV-2 IgG/IgM Rapid Qualitative Test produced by Biotime Biotechnology and published the analysis report “Internal validation of a CE marked in vitro diagnostic use anti-body test against PCR and local clinical-radiological criteria for COVID-19 and comparison with manufacturer proposed performance characteristics. ” The report indicates that the verification result was the same as the product performance claimed by Biotime Biotechnology.
This verification adopted the Biotime product to retest and evaluate 62 inpatient samples. Among them, 52 were positive confirmed by PCR testing and clinical diagnosis and 10 were healthy samples. The result is shown as below:
① The positive detection rate of Biotime reagents is 96.2% and negative rateis100% (see Figure 1)
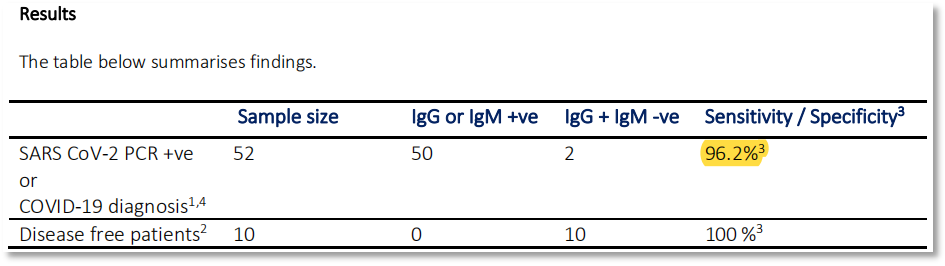
Figure 1
② As for 2 false negative results: Because the control line was unclear, the report considered that they might be caused by operation error or invalid test.(see Figure 2)

Figure 2
③The report also indicated that PCR testing exists 30% missed inspection risk in COVID-19 diagnosis. Therefore, IgM or IgG/IgM antibody testing plays an important role in acute patient group besides PCR.(see Figure 3)

Figure 3
④At last, the report mentioned that, positive results were shown in both IgG and IgM though the number of samples were relevant small, which could guarantee the effectiveness of all verification results.(see Figure 4)

Figure 4
© 2016 Xiamen Biotime Biotechnology Co., Ltd. all rights reserved